4D-Glycosylation Proteomics Service
Based on a high-resolution mass spectrometry platform combined with ion mobility separation technology, MtoZ Biolabs has launched the 4D-glycosylation proteomics service which enables systematic detection and quantification of N-glycosylation, O-glycosylation, and other glycan modifications. Through efficient glycopeptide enrichment and optimized experimental workflows, this service can precisely identify glycosylation sites, capture low-abundance modifications, and analyze their distribution and abundance changes. The final output includes site identification, quantitative differences, and functional annotation data, helping researchers to map a complete glycosylation profile, supporting studies in cellular function, signaling pathway analysis, and biomarker screening.
Overview
Glycosylation refers to the covalent attachment of glycans to amino acid residues after protein synthesis, thereby regulating protein folding, stability, localization, and intermolecular interactions. Glycosylation is extensively involved in biological processes such as cell signaling, immune recognition, molecular transport, and metabolic regulation, and is of great importance for maintaining physiological functions and responding to external stimuli. Glycosylation proteomics analysis can achieve systematic detection and precise characterization of multiple types of glycosylation modifications, providing systematic data on glycosylation site distribution, abundance changes, and functional annotations. It is widely applied in cellular function analysis, immunological research, signaling network construction, and potential biomarker discovery, offering reliable support for a deeper understanding of the regulatory mechanisms of glycosylation in life activities.
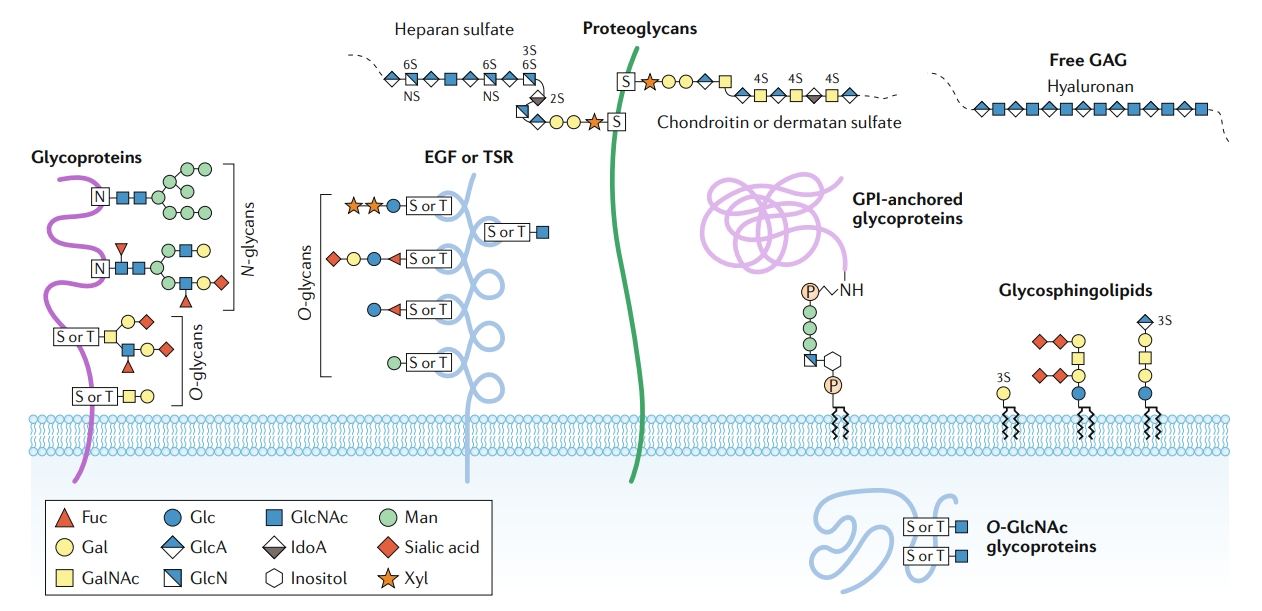
Reily, C. et al. Nature Reviews Nephrology, 2019.
Figure 1. Major Types of Glycosylation in Humans.
Analysis Workflow
1. Sample Preparation and Protein Extraction
Samples are subjected to protein extraction and pretreatment according to experimental requirements to ensure detection stability.
2. Protein Digestion and Glycopeptide Enrichment
Enzymatic digestion is performed under optimized conditions, and specific enrichment methods are applied to capture glycosylated peptides.
3. 4D Mass Spectrometry Detection
Relying on high-resolution LC-MS/MS combined with ion mobility separation, precise identification and quantification of glycosylation sites are achieved.
4. Data Analysis
Database searching and multidimensional bioinformatics analysis are employed to output glycosylation site distribution, quantitative differences, and functional annotations.
Sample Submission Suggestions
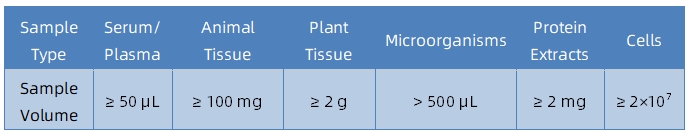
Service Advantages
1. High-Resolution Detection
Relying on the 4D mass spectrometry platform, it enables precise capture of low-abundance glycosylation modifications in complex samples.
2. Comprehensive Modification Coverage
Supports systematic identification and quantification of N-glycosylation, O-glycosylation, and multiple types of modifications.
3. Sensitivity and Reproducibility
Combining specific enrichment and optimized workflows, it enhances detection sensitivity and ensures data consistency.
4. Customized Solutions
Analytical strategies can be flexibly adjusted according to sample characteristics and research objectives to meet diverse needs.
Applications
1. Immunological Research
The 4D-glycosylation proteomics service can be used to analyze the role of glycosylation in immune recognition and antibody function, supporting immunology-related studies.
2. Cellular Function Analysis
Systematic detection of glycosylation modifications helps to study regulatory mechanisms in cellular biological processes.
3. Signaling Pathway Research
The 4D-glycosylation proteomics service can be used to monitor the effects of glycosylation modifications on key signaling molecules and pathway activities.
4. Biomarker Screening
By screening glycosylation modification sites associated with specific states, potential functional biomarkers can be identified.
FAQ
Q1: Can N-Glycosylation and O-Glycosylation Be Detected Simultaneously?
A1: Yes. Through diverse glycopeptide enrichment strategies, 4D detection can cover N-glycosylation, O-glycosylation, and other related modifications, enabling systematic identification and quantification at a global level.
Q2: Does the Heterogeneity of Complex Glycans Affect Detection?
A2: The high structural diversity of glycans does increase the difficulty of analysis, but with specific enzymatic digestion, optimized separation strategies, and ion mobility separation, glycan heterogeneity can be resolved to a large extent, ensuring comprehensive and accurate results.
Q3: How to Address the Challenge of Detecting Low-Abundance Glycosylation Modifications?
A3: By applying specific enrichment strategies (such as HILIC or lectin enrichment), multidimensional separation, and the high sensitivity of 4D mass spectrometry, the detection rate of low-abundance glycopeptides can be significantly improved.







