S-prenylation Analysis Service
S-prenylation is a critical lipid modification in which isoprenoid groups are covalently attached to cysteine residues of proteins through thioether linkages. This modification enhances the hydrophobicity of proteins, facilitates membrane association, and regulates diverse signaling pathways. Increasing evidence shows that S-prenylation is indispensable for protein localization, stability, and functional activity across eukaryotes and some viruses.
MtoZ Biolabs offers a specialized S-prenylation Analysis Service that integrates chemical enrichment, high-resolution LC-MS/MS, and bioinformatics interpretation. Our platform provides both targeted protein analysis and proteomics-based profiling, enabling researchers to uncover the functional significance of S-prenylation in physiology and disease. Our flexible analytical solutions can be tailored to different project needs:
🔸Targeted Protein S-prenylation Analysis
We focus on specific proteins of interest, using selective enrichment, immunoprecipitation, and LC-MS/MS to identify S-prenylation sites and quantify modification levels under various experimental conditions. This approach is suitable for hypothesis-driven studies where defined targets are being evaluated.
🔸S-prenylation Proteomics Profiling
For broader investigations, we conduct large-scale discovery of prenylated proteins across entire proteomes. Our workflows capture canonical CaaX-motif proteins and novel substrates, providing a comprehensive landscape of prenylation events. Combined with bioinformatics annotation, this approach links modification patterns to pathways, networks, and disease relevance.
What is S-prenylation?
Protein S-prenylation refers to the covalent addition of hydrophobic isoprenoid groups, most commonly farnesyl (C15) or geranylgeranyl (C20), to cysteine residues located near the C-terminus of proteins. This process is catalyzed by prenyltransferases, including farnesyltransferase (FTase) and geranylgeranyltransferases (GGTase I and II). Substrate proteins often contain a CaaX motif, where C represents cysteine, a stands for aliphatic residues, and X determines substrate specificity. After the initial prenylation, further modifications such as proteolytic cleavage of the –aaX tripeptide and carboxymethylation of the cysteine residue may occur, enhancing membrane anchoring. These steps collectively increase protein hydrophobicity and facilitate their interaction with membranes and signaling complexes.
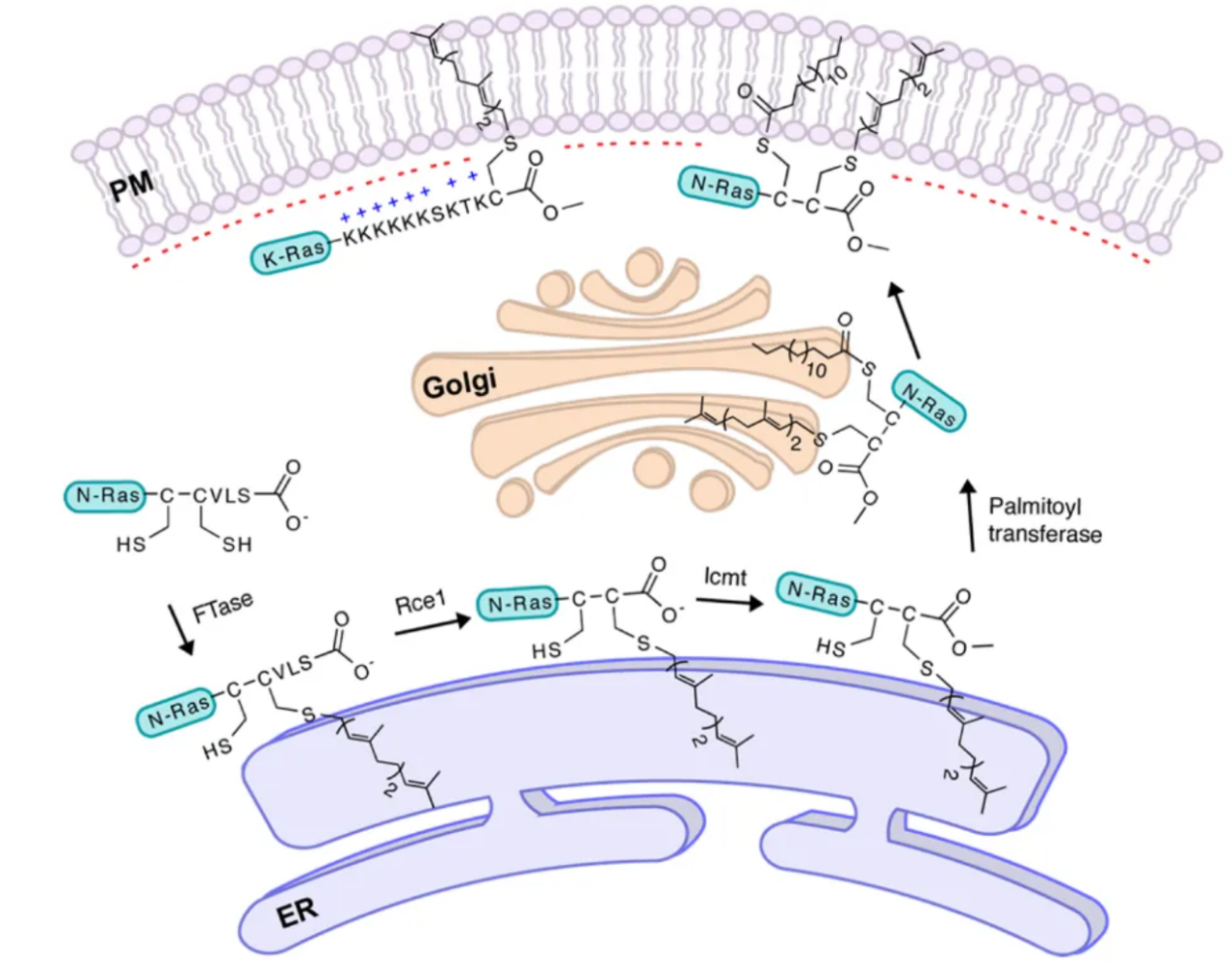
Palsuledesai, CC. et al. ACS Chem Biol. 2015.
Figure 1. The Process of Protein Prenylation
S-prenylation plays an essential role in regulating small GTPases, including Ras, Rho, and Rab family proteins. These proteins are master regulators of cell growth, differentiation, motility, and vesicle trafficking. Dysregulation of S-prenylation has been directly linked to multiple human diseases, including cancers, cardiovascular disorders, infectious diseases, and neurodegenerative conditions. Ongoing research is expanding our understanding of S-prenylation dynamics, substrate diversity, and functional cross-talk with other post-translational modifications. This highlights the importance of advanced technologies that can precisely map S-prenylation sites and quantify modification changes in different biological contexts.
Analysis Workflow
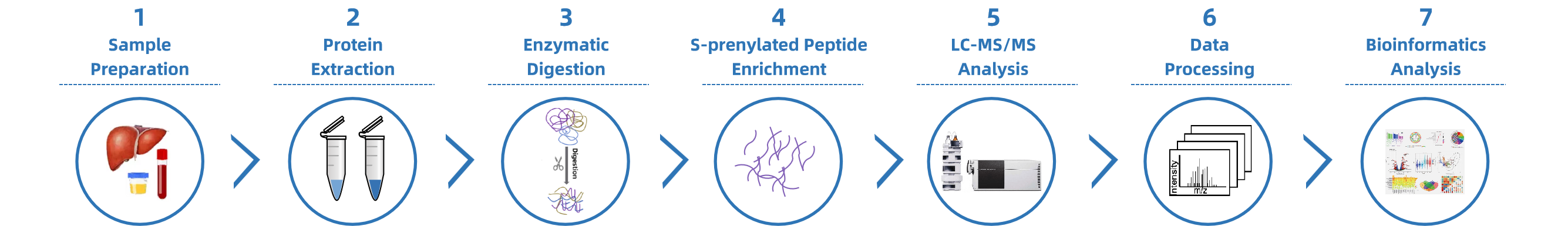
Sample Submission Suggestions
1. Sample Types: Acceptable samples include cultured cells, tissue lysates, body fluids, purified proteins, or other biological samples.
2. Protein Amount: At least 5-10 mg of total protein is recommended for comprehensive analysis.
3. Storage and Shipping: Samples should be flash-frozen in liquid nitrogen and shipped on dry ice.
4. Replicates: At least 3 biological replicates are recommended for statistical reliability.
*Note: If you have special sample types or require additional guidance, please contact us for personalized support before sample preparation.
Service Advantages
By choosing MtoZ Biolabs, clients benefit from:
☑️Cutting-Edge Mass Spectrometry Platforms delivering high-resolution data for confident identification of low-abundance modifications.
☑️Tailored Service Options including targeted assays and global proteomics, adaptable to specific research questions.
☑️High Sensitivity and Specificity through optimized enrichment and data acquisition strategies.
☑️Expert Scientific Team with extensive experience in post-translational modification analysis.
☑️Robust Bioinformatics Support providing functional annotation, pathway analysis, and systems-level integration.
☑️Efficient Turnaround Time ensuring timely delivery of high-quality results.
FAQ
Q1: Can this service distinguish between farnesylation and geranylgeranylation?
Yes. Using high-resolution LC-MS/MS combined with optimized enrichment strategies, MtoZ Biolabs can differentiate between farnesylated and geranylgeranylated peptides based on precise mass shifts and fragmentation patterns.
Q2: Do you provide cross-talk analysis of S-prenylation with other PTMs?
Yes. Bioinformatics pipelines can integrate prenylation data with phosphorylation, acetylation, or ubiquitination datasets, helping to uncover multi-layered regulatory mechanisms.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment
4. Data Analysis, Preprocessing, and Estimation
5. Bioinformatics Analysis
6. Raw Data Files
By offering precise analytical workflows, MtoZ Biolabs helps researchers turn complex prenylation patterns into mechanistic insights that advance biomedical science. Contact us to explore tailored solutions for your research.







