Glycopeptide Analysis Service
MtoZ Biolabs provides Glycopeptide Analysis Service that integrates optimized sample preparation, targeted enrichment strategies, and high-resolution LC-MS/MS platforms to deliver comprehensive characterization of protein glycosylation. Our solutions include glycopeptide identification, glycosylation site mapping, structural elucidation of glycan compositions and linkages, and quantitative profiling of glycoforms. By combining advanced instrumentation with expert bioinformatics pipelines, we ensure accurate, reproducible, and regulatory-compliant data that support both academic research and biopharmaceutical development.
Overview
Glycopeptides, generated by proteolytic digestion of glycoproteins, are unique analytical targets because they preserve both the peptide backbone and the covalently attached glycans. This dual information provides site-specific insight into protein glycosylation, linking glycan structures to their corresponding protein regions.
The analysis of glycopeptides is essential in biomedical and pharmaceutical research. In biopharmaceutical development, glycosylation is considered a critical quality attribute under regulatory guidelines such as ICH Q6B, as it directly affects drug safety, efficacy, and pharmacokinetics. Abnormal glycosylation patterns have also been associated with cancers, autoimmune disorders, neurodegenerative diseases, and infectious diseases. Therefore, detailed characterization of glycopeptides is vital not only for fundamental biology but also for clinical and therapeutic applications.

de Haan N. et al. Nat Rev Chem. 2020.
Figure 1. Glycopeptide Analysis Workflow
Analysis Workflow
The general analytical workflow for the Glycopeptide Analysis Service is as follows:
1. Sample Preparation and Quality Control
Proteins from biological or pharmaceutical samples are evaluated for concentration and purity. Buffer exchange or cleanup is performed when necessary to ensure compatibility with downstream analysis.
2. Enzymatic Digestion
Proteins are digested with proteases such as trypsin, or combinations of enzymes, to generate peptides and glycopeptides while preserving glycosylation integrity.
3. Glycopeptide Enrichment
Selective enrichment methods, such as lectin affinity, HILIC, are applied to increase the relative abundance of glycopeptides and reduce interference from non-glycosylated peptides.
4. LC-MS/MS Analysis
Enriched glycopeptides are analyzed using high-resolution LC-MS/MS platforms to reveal both glycan structures and peptide backbones.
5. Data Processing and Bioinformatics
Spectral data are interpreted using curated glycan databases and specialized software to assign glycan compositions, localize glycosylation sites, and quantify glycoform distributions.
6. Reporting
Clients receive a comprehensive report including experimental details, annotated glycopeptide lists, glycosylation site assignments, structural information, quantitative data, and interpretation.
Service Advantages
1. Advanced Analysis Platform
MtoZ Biolabs established an advanced Glycopeptide Analysis Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
2. Comprehensive Analytical Coverage
From glycopeptide identification and glycosylation site mapping to structural elucidation and quantitative profiling, we provide end-to-end solutions.
3. Expert Bioinformatics Integration
Curated glycan databases and specialized algorithms deliver confident assignments, reproducible quantification, and biologically meaningful interpretation.
4. Regulatory and Application-Oriented Outputs
Standardized workflows and strict quality control provide reproducible results aligned with academic publishing requirements and regulatory guidelines for biopharmaceutical development.
Sample Submission Suggestions
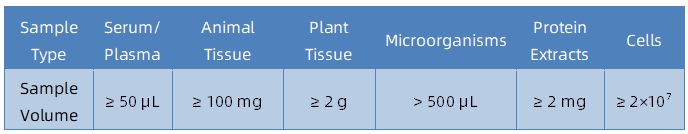
It is recommended to contact our technical support team before submitting samples to determine sample suitability and obtain tailored submission guidelines.
Applications
Practical applications of Glycopeptide Analysis Service are as follows:
1. Monoclonal Antibody Development
Characterize Fc glycosylation patterns that influence antibody-dependent cellular cytotoxicity (ADCC), pharmacokinetics, and immunogenicity.
2. Biosimilarity Assessment
Compare glycopeptide profiles between reference and biosimilar products to demonstrate structural equivalence and meet regulatory requirements.
3. Vaccine Glycoprotein Characterization
Analyze glycosylation of viral antigens to inform immunogenicity, vaccine design, and stability studies.
4. Site-Directed Mutagenesis Studies
Confirm changes in glycosylation occupancy and structure following mutagenesis of glycosylation sites.
5. Process Monitoring and Batch Consistency
Evaluate glycosylation heterogeneity across manufacturing batches as a critical quality attribute in biopharmaceutical production.
FAQ
Q1: Can Both N-linked and O-linked Glycopeptides Be Analyzed?
A1: Yes. Our enrichment strategies, including lectin affinity, HILIC, and hydrazide chemistry, in combination with LC-MS/MS, enable sensitive and specific analysis of both N-linked and O-linked glycopeptides.
Q2: Can the Service Provide Quantitative Glycoform Distribution Data?
A2: Yes. Relative quantification of glycoforms is performed using specialized bioinformatics pipelines, enabling comparison of glycosylation patterns across biological conditions, production batches, or therapeutic candidates.







