Protein Glycosylation Analysis Service
Based on a high-resolution LC-MS/MS mass spectrometry platform combined with high-performance liquid chromatography separation, MtoZ Biolabs has launched the protein glycosylation analysis service which enables systematic identification and quantification of glycosylation sites in protein samples, as well as analysis of glycan structural types, modification distribution, and abundance changes. The final output data not only include precise identification results of glycosylation sites and quantitative difference information, but also cover glycan structure annotation and potential functional interpretation, providing researchers with reliable data support for revealing protein functional regulation and complex biological processes.
Overview
Protein glycosylation refers to the process in which glycans are covalently attached to specific sites of proteins through enzymatic reactions, and it is one of the most common and functionally diverse post-translational modifications. It can regulate protein folding, stability, and secretion, and influence molecular interactions and signal transduction processes. The principle of protein glycosylation analysis mainly relies on high-resolution mass spectrometry technology, combined with specific enrichment strategies and enzymatic methods, to achieve precise identification and quantification of glycan structures, site distribution, and modification levels. This method has been widely applied in structural biology, epigenetics, cell communication, and biomarker research, providing important data support for in-depth elucidation of cellular functional networks and molecular regulatory mechanisms.
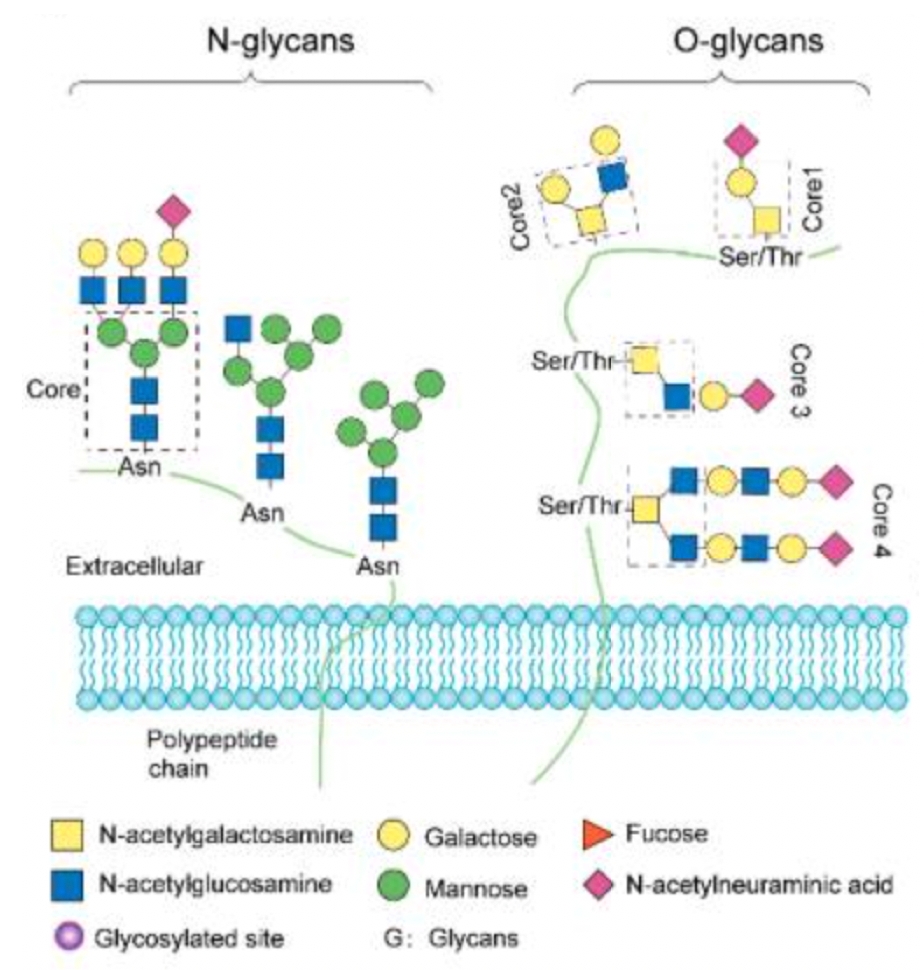
Fang, P. et al. International Journal of Molecular Sciences, 2022.
Figure 1. Common Types of Glycosylation Modifications.
Services at MtoZ Biolabs
1. Target Protein Glycosylation Analysis
MtoZ Biolabs conducts site-specific glycosylation profiling for designated target proteins, including identification of glycosylation sites, glycan types, and microheterogeneity patterns. Leveraging high-resolution LC-MS/MS, we can quantitatively compare glycosylation changes under different experimental conditions, helping researchers clarify how glycan modifications influence target protein structure and function.
2. Glycosylation Proteomics Analysis
MtoZ Biolabs integrates commonly used glycopeptide enrichment strategies such as HILIC and lectin-based enrichment with advanced mass spectrometry systems to achieve large-scale identification and quantification of glycosylated peptides in complex samples. This analysis reveals global glycosylation regulatory patterns and supports pathway investigation, glycan structural assessment, and comprehensive glycoproteome mapping.
Analysis Workflow
1. Sample Preparation
Protein extraction and quantification are performed on cell, tissue, or body fluid samples to ensure sample quality and uniformity.
2. Protein Digestion
Proteins are digested into peptides to facilitate the detection and analysis of glycosylation modifications.
3. Glycopeptide/Glycan Enrichment
HILIC, lectin, or specific antibody methods are used for the selective enrichment of glycopeptides or glycans to improve detection sensitivity.
4. Mass Spectrometry Detection
The enriched products are analyzed by a high-resolution LC-MS/MS platform for precise identification and quantification.
5. Data Analysis
Databases and bioinformatics tools are used to annotate glycosylation sites and glycan structures, providing distribution characteristics, modification levels, and functional information.
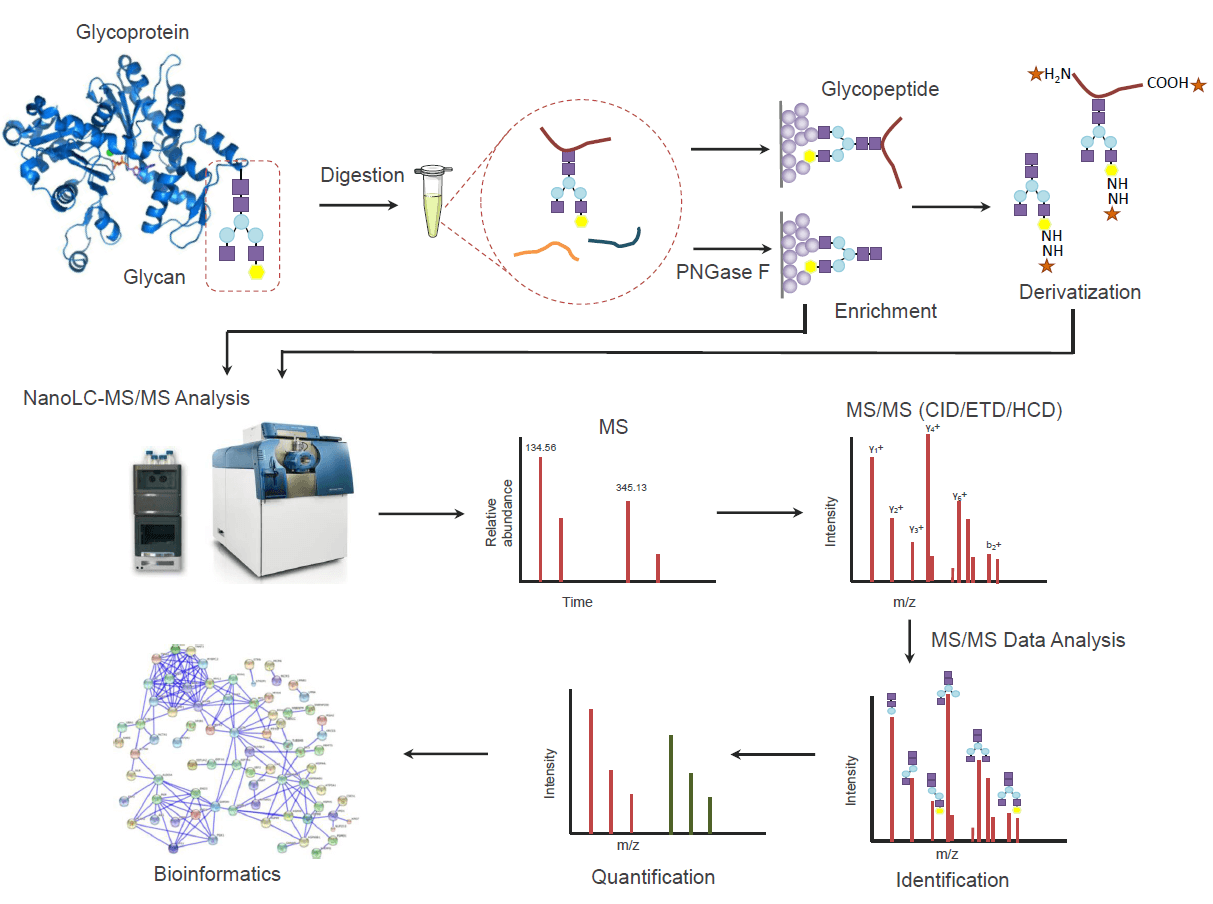
Figure 2. The Workflow of Protein Glycosylation Analysis.
Sample Submission Suggestions
1. Sample Type and Quantity
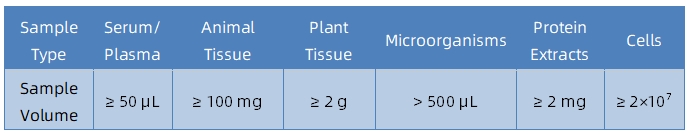
2. Sample Storage
Samples should be stored under low-temperature conditions (such as at -80°C) to avoid repeated freeze-thaw cycles, in order to prevent protein degradation and the loss of glycosylation modifications.
3. Sample Transportation
It is recommended to use dry ice or cold chain transportation to ensure that the samples maintain structural stability and modification integrity before reaching the testing platform.
Service Advantages
1. High-Sensitivity Detection
Relying on a high-resolution LC-MS/MS platform and optimized workflows, low-abundance glycosylation sites can be accurately captured in complex backgrounds, ensuring the reliability of results.
2. Specific Enrichment
By combining HILIC, lectin, and glycan-specific methods, glycopeptides or glycans can be effectively separated, improving signal-to-noise ratio and detection accuracy.
3. Professional Team Support
Experiments and data interpretation are conducted by experts with extensive experience in mass spectrometry analysis and glycosylation research, ensuring standardized procedures and trustworthy results.
4. Customized Solutions
The analysis range and depth can be flexibly adjusted according to different research needs, providing targeted glycosylation data support.
Applications
1. Structural Biology Research
Through the analysis of glycosylation sites and glycan structures, the spatial conformation and folding characteristics of proteins can be revealed, providing key data for structural elucidation.
2. Molecular Interaction Exploration
The protein glycosylation analysis service can be used to study its regulatory role in protein-protein and protein-receptor interactions.
3. Cell Communication Research
By detecting the glycosylation characteristics of membrane proteins and secreted proteins, their functions in cell recognition, signal transmission, and immune regulation can be analyzed.
4. Quality Control and Bioengineering
Protein glycosylation analysis service can be applied to the detection of glycosylation modifications in recombinant proteins or antibodies, assessing their consistency and stability, and supporting process optimization.
FAQ
Q1: Can Glycan Structures Be Resolved?
A1: Yes. Through LC-MS/MS combined with glycan-specific separation methods, it is possible not only to identify glycosylation sites but also to annotate glycan composition and partial structural features.
Q2: Can "Site and Glycan" Joint Identification Be Achieved at the Glycopeptide Level?
A2: Yes. By using parallel fragmentation strategies such as HCD/ETD, peptide backbone ions and glycosidic bond-retaining ions can be obtained simultaneously, enabling the determination of both site and glycan composition while improving localization confidence.
Q3: Can the Results be Integrated with Proteomics/Other PTMs for Interpretation?
A3: Yes. The service supports integration with whole-proteome quantification and other PTMs such as phosphorylation or acetylation, enabling comprehensive analysis at the levels of site co-occurrence, networks, and pathways.







