S-Glutathionylation Mapping Service
Based on a high-resolution mass spectrometry (LC-MS/MS) platform and highly specific chemical labeling technology, MtoZ Biolabs has launched the S-glutathionylation mapping service to systematically analyze S-glutathionylation modifications in proteins. This service combines differential labeling, affinity enrichment, and quantitative mass spectrometry detection to achieve precise identification and abundance measurement of modification sites. Through database comparison and bioinformatics analysis, it provides information on site distribution, dynamic changes, and related functions of S-glutathionylation modifications, offering high-confidence data support for redox regulation and protein function research.
What Is S-Glutathionylation?
Protein S-glutathionylation is a reversible cysteine-based redox modification in which a mixed disulfide bond (PSSG) is formed on cysteine residues of proteins, regulating their conformation, stability, and activity. This modification plays a crucial role in maintaining cellular redox homeostasis, protecting against oxidative stress, and modulating signal transduction. This service reveals the distribution and variation patterns of such modifications and is widely applied in oxidative stress research, metabolic regulation, drug mechanism studies, and protein function analysis.
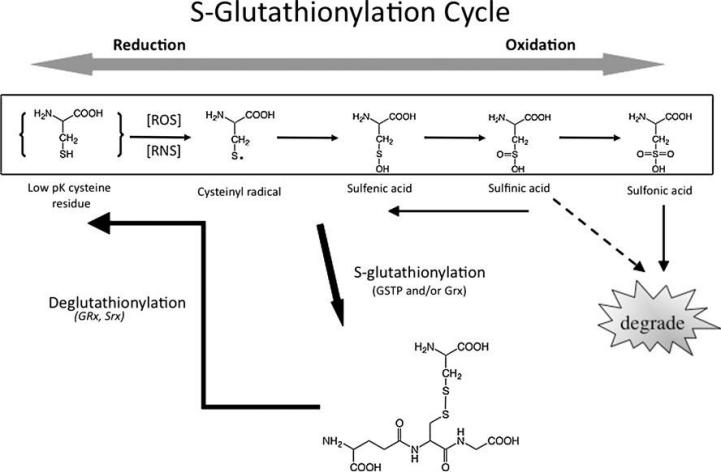
Xiong, Y. et al. Antioxidants & Redox Signaling, 2011.
Figure 1. S-glutathionylation Cycle.
Analysis Workflow
1. Protein Extraction and Sample Preparation
Total proteins are extracted from samples under antioxidant conditions to preserve the native state of S-glutathionylation modifications.
2. Chemical Labeling and Enrichment
Specific chemical labeling or affinity capture strategies are applied to selectively enrich peptides containing S-glutathionylation modifications.
3. Mass Spectrometry Detection and Data Acquisition
High-resolution LC-MS/MS is used to detect modified peptides and acquire fragmentation spectra for precise site characterization.
4. Data Analysis and Reporting
Database comparison and algorithmic analysis are performed to identify modification sites, quantify abundance changes, and provide functional annotations, followed by the generation of a standardized report.
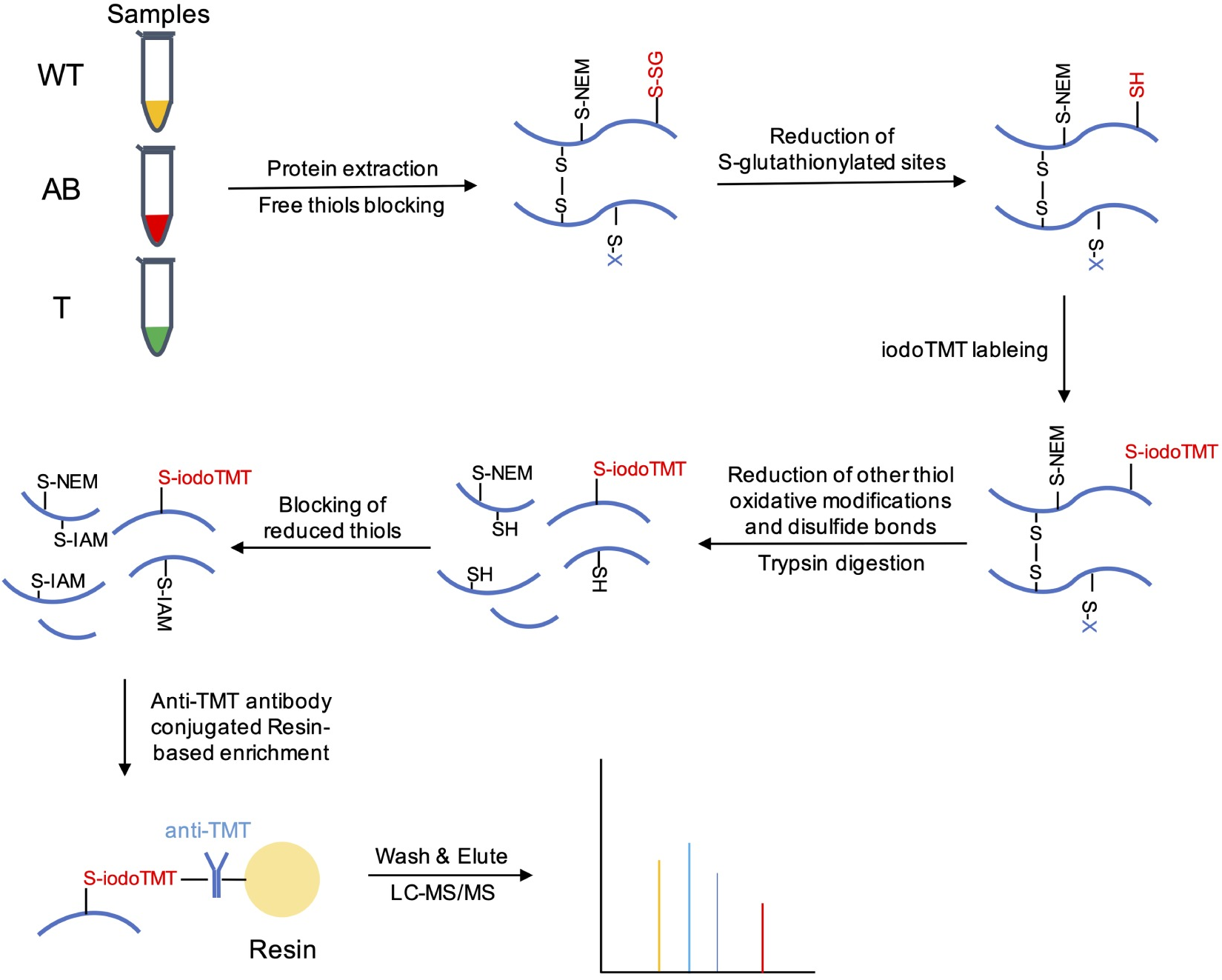
Li, Z Y. et al. PLOS Pathogens, 2020.
Figure 2. Schematic Overview of Isotopic-Labeling Strategy to Identify and Quantify S-Glutathionylated Proteins.
Sample Submission Suggestions
1. Sample Type
Cells, tissues, serum, plasma, and microbial samples are acceptable. Protein extracts or lyophilized powders are also supported.
Note: Strong oxidants or reductants should be avoided during sample handling to preserve the native S-glutathionylation state.
2. Sample Storage
Samples should be tightly sealed and protected from light at -80°C for long-term storage, or -20°C for short-term storage. Repeated freeze-thaw cycles and prolonged air exposure should be avoided to prevent modification degradation.
3. Sample Transportation
Liquid samples should be shipped under cold-chain conditions. Lyophilized samples can be shipped at room temperature for short periods but should be protected from high temperature, humidity, and strong light to ensure modification stability and analytical accuracy.
Service Advantages
1. High-Resolution Detection Platform
Based on advanced LC-MS/MS systems, enabling highly sensitive detection and precise quantification of S-glutathionylation modifications.
2. High-Specificity Enrichment Strategy
Utilizes specific labeling and affinity capture techniques to enhance the identification efficiency of modified peptides.
3. Standardized Analytical Workflow
Implements rigorous quality control throughout the entire process to ensure accurate and reliable results.
4. Customized Research Design
Offers flexible experimental strategies tailored to specific research objectives, meeting diverse analytical needs.
Applications
1. Redox Regulation Studies
The S-glutathionylation mapping service can be used to analyze protein modification changes under different oxidative stress conditions, revealing the regulatory mechanisms of redox balance.
2. Metabolic Pathway Analysis
By detecting S-glutathionylation modifications of metabolic enzymes, it enables the evaluation of their regulatory roles in energy metabolism and material conversion processes.
3. Protein Function Research
Through precise site identification, the service helps explore the impact of S-glutathionylation on protein structure, activity, and complex formation.
4. Environmental Stress Response Studies
The S-glutathionylation mapping service can monitor modification dynamics in cells or tissues exposed to oxidative, heavy metal, or radiation stress conditions.
FAQ
Q1: How Can Modification Loss Be Prevented during Sample Preparation?
A1: It is recommended to operate under low-temperature, dark, and antioxidative conditions, avoiding the use of strong oxidants or reductants (such as DTT or β-ME), to preserve the native S-glutathionylation state.
Q2: Can Quantitative Analysis of Modification Sites Be Performed?
A2: Yes. MtoZ Biolabs combines TMT or Label-Free quantification strategies to compare S-glutathionylation levels across different treatment groups.
Q3: How Can S-Glutathionylation Be Distinguished from Other Cysteine Redox Modifications?
A3: Through specific chemical labeling and high-resolution LC-MS/MS detection, S-glutathionylation can be accurately differentiated from other modifications such as S-nitrosylation or disulfide bond formation.







