S-Nitrosylation Analysis Service
S-Nitrosylation is a reversible post-translational modification in which nitric oxide (NO) covalently attaches to the thiol group of cysteine residues, forming S-nitrosothiols. Acting as a redox-based molecular switch, this modification fine-tunes protein activity, stability, and interaction dynamics, thereby influencing cellular signaling, stress responses, and disease progression.
MtoZ Biolabs offers a complete S-Nitrosylation Analysis Service designed to meet the needs of both focused and large-scale studies. Utilizing advanced enrichment chemistries such as the Biotin-Switch Assay and Resin-Assisted Capture (RAC), combined with high-resolution LC–MS/MS and bioinformatics analysis, we deliver precise and biologically meaningful data for both focused and large-scale studies. Clients only need to submit their samples, and we will manage the entire workflow, from protein extraction and identification to quantitative evaluation and functional annotation, delivering a panoramic view of nitrosylation across proteins and pathways.
1. Target Protein S-Nitrosylation Analysis
This service is tailored for the in-depth investigation of nitrosylation on a specific protein of interest. MtoZ Biolabs accurately identifies S-nitrosylated cysteine residues and quantifies their modification levels under distinct physiological or experimental conditions. This approach is ideal for validating the functional relevance of nitrosylation in key signaling enzymes, transcriptional regulators, or redox-sensitive proteins.
2. S-Nitrosylation Proteomics
For broader discovery studies, MtoZ Biolabs offers S-nitrosylation proteomics, enabling large-scale profiling of nitrosylated proteins within complex biological systems. We systematically map S-nitrosylation sites, compare modification dynamics across treatment groups, and perform downstream analyses such as pathway enrichment and protein-interaction network construction. This global approach uncovers how nitrosylation reshapes signaling circuits and contributes to disease mechanisms at the systems level.
What is S-Nitrosylation?
S-nitrosylation represents one of the most important redox-based post-translational modifications. This process involves the covalent attachment of an NO group to the thiol side chain of cysteine residues, forming S-nitrosothiols (SNOs). S-nitrosylation is dynamic and reversible. The modification can be propagated by transnitrosylation, where the NO group is transferred from one protein thiol to another. Conversely, it can be reversed through denitrosylation mechanisms mediated by glutathione and the S-nitrosoglutathione reductase (GSNO-R) system. This tight regulation ensures that nitrosylation functions as a redox-sensitive molecular switch in diverse cellular contexts.
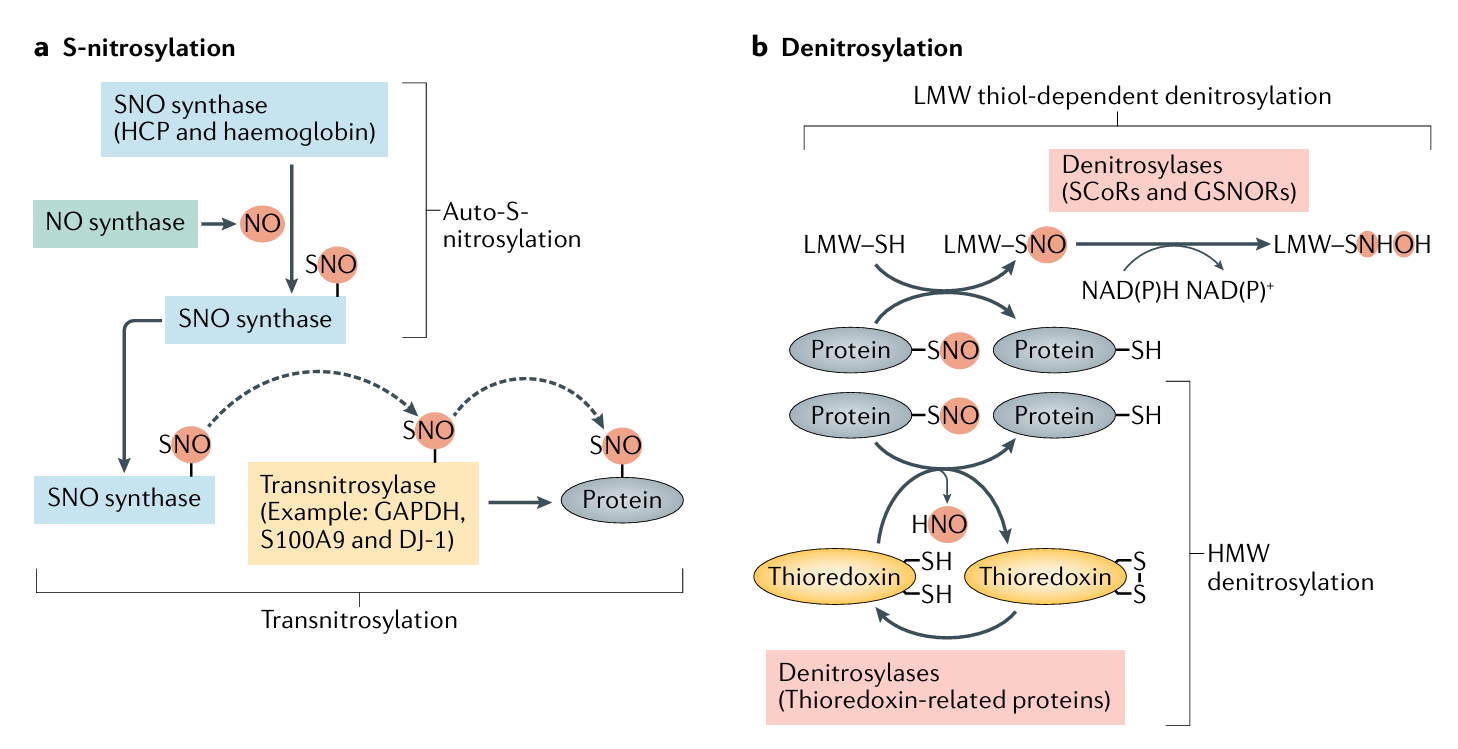
Zhou, H. L. et al. Nat Rev Endocrinol. 2022.
Figure 1. Working Model of Protein S-nitrosylation and Denitrosylation
The biological importance of S-nitrosylation was first highlighted through discoveries of nitrosylated hemoglobin, which regulates oxygen transport, and nitrosylated caspase proteins, which influence apoptosis. Since then, proteomic studies have revealed that S-nitrosylation is widespread across many protein families, including metabolic enzymes, transcription factors, and receptors. Functionally, it regulates vascular tone, immune responses, neurotransmission, and metabolic adaptation. Dysregulated S-nitrosylation has been implicated in a wide range of human diseases, including cardiovascular disorders, cancer, and neurodegenerative diseases. This makes S-nitrosylation analysis essential for understanding disease mechanisms, discovering biomarkers, and developing therapeutic strategies.
Analysis Workflow
1. Samples are lysed and proteins extracted under optimized conditions.
2. Free thiol groups are chemically blocked to avoid non-specific reactions.
3. S-nitrosylated cysteine residues are selectively reduced by ascorbic acid.
4. Reduced thiols are labeled with biotin to generate stable derivatives.
5. Proteins are digested into peptides and biotinylated peptides are enriched.
6. Enriched peptides are analyzed using high-resolution LC-MS/MS.
7. Raw data are processed for peptide identification, site localization, quantification, and functional annotation.
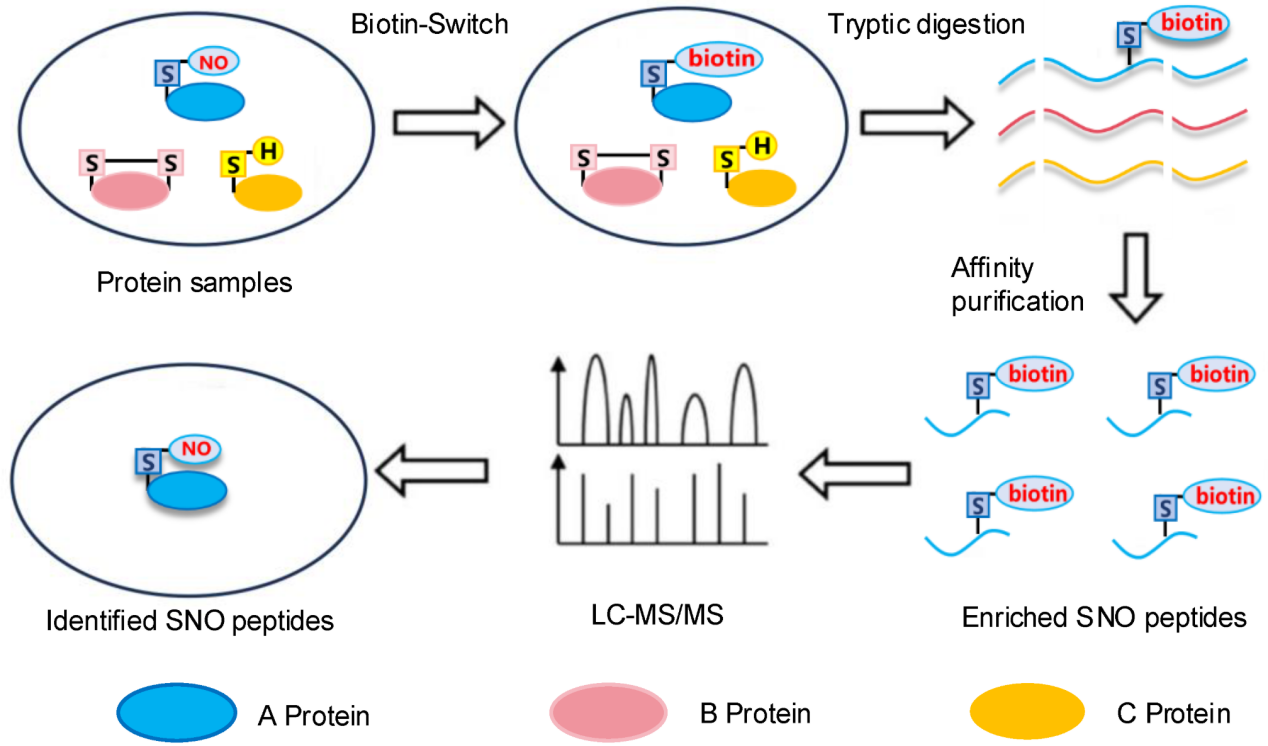
Lin, W. et al. Curr Issues Mol Biol. 2025.
Figure 2. Schematic Illustration of the Procedure of S-Nitrosylation Analysis by LC-MS/MS
Sample Submission Suggestions
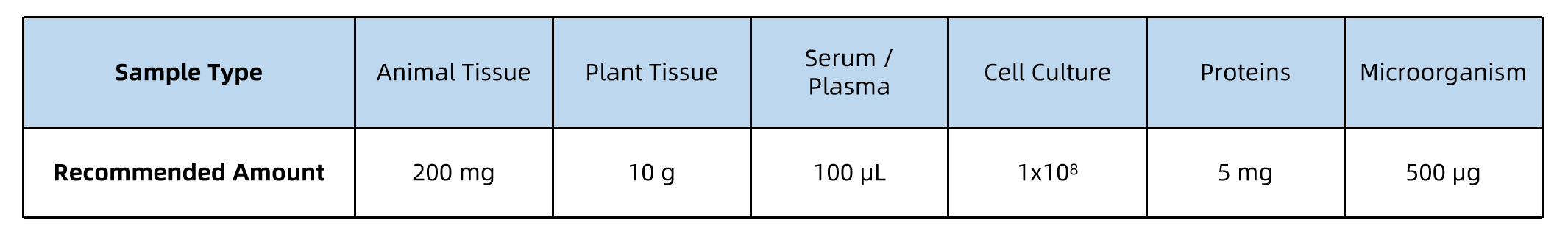
🔸Storage and Shipping: Samples should be stored at -80℃ and shipped with dry ice.
🔸Replicates: At least 3 biological replicates are recommended for statistical reliability.
*Note: If you have special sample types or require additional guidance, please contact us for personalized support before sample preparation.
Service Advantages
☑️Optimized workflows to preserve labile S-nitrosylation.
☑️High-resolution LC-MS/MS ensures accurate site identification.
☑️Customizable service options to meet specific research needs.
☑️Experienced scientific team with strong expertise in PTM analysis.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment
4. Data Analysis, Preprocessing, and Estimation
5. Bioinformatics Analysis
6. Raw Data Files
If you are interested in our S-Nitrosylation Analysis Service, please feel free to contact us. Our technical specialists are available to provide a free business assessment.







