Targeted Post-Translational Modification (PTM) Profiling Service
MtoZ Biolabs provides Targeted Post-Translational Modification (PTM) Profiling Services tailored to specific proteins of interest, enabling accurate detection, localization, and quantification of key modifications such as phosphorylation, acetylation, methylation, ubiquitination, and nitration. These services support protein characterization, therapeutic development, and product quality assurance.
What Is Post-Translational Modification (PTM)?
Post-Translational Modification (PTM) is the covalent and enzyme-mediated alteration of proteins after translation, affecting functional groups, amino acid side chains, termini, or entire domains. These modifications regulate protein stability, activity, localization, interactions, and signaling pathways.
Common Types of Post-Translational Modifications
1. Phosphorylation: Addition of phosphate groups to serine, threonine, or tyrosine residues, influencing signal transduction and enzyme activity.
2. Acetylation: The addition of acetyl groups, usually on lysine residues, involved in regulating gene expression and protein stability.
3. Methylation: The addition of methyl groups to lysine or arginine residues, playing a critical role in gene regulation and chromatin structure.
4. Ubiquitination: Attachment of ubiquitin molecules to proteins, marking them for degradation or influencing protein-protein interactions.
These modifications are essential for regulating protein function in normal cellular processes and in response to changes in the cellular environment.
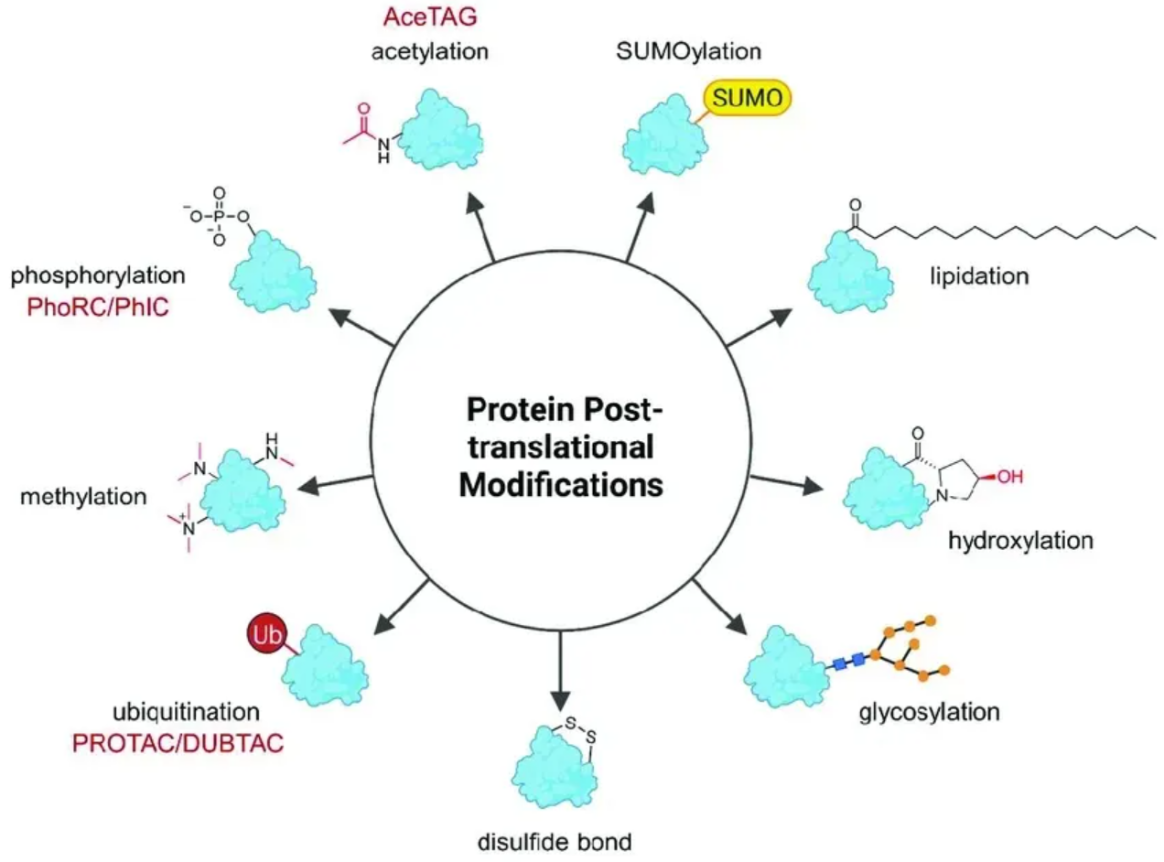
Heitel, P. et al. Molecules. 2023.
Figure 1. Common Types of Protein Post-Translational Modifications
Targeted Post-Translational Modification (PTM) Profiling involves analyzing a defined protein (or set of proteins) rather than undertaking broad global proteomics. It focuses on specific PTM types, sites, and conditions in proteins of interest, delivering high-confidence data on modification states, site occupancy, and dynamic changes.
Principle of Targeted Post-Translational Modification (PTM) Profiling
MtoZ Biolabs employs advanced mass spectrometry methods and enrichment strategies to localize and quantify PTMs with precision.
1. Enrichment of Modified Peptides
Specific PTMs are enriched using affinity or chemical capture methods (for example TiO₂ for phosphopeptides, anti-acetyl-lysine antibodies for acetylation, K-ε-GG antibodies for ubiquitination).
2. High-Resolution LC-MS/MS Analysis
Nano-liquid chromatography is coupled with orbitrap or Q-TOF mass spectrometers to identify and quantify peptides carrying the modification, determining exact site and modification stoichiometry.
3. Quantitative Strategies
Label-free or isotopic labeling (SILAC, TMT) approaches enable quantitative comparison between conditions or protein lots.
4. Data Interpretation and Validation
Specialized software and manual validation confirm site localization, modification type, and relative abundance, ensuring robust and reproducible results.
Targeted Post-Translational Modification (PTM) Profiling Service at MtoZ Biolabs
MtoZ Biolabs offers a comprehensive suite for Targeted PTM Profiling focusing on client-specified proteins. Our services include:
💠Targeted PTM Site Mapping: Identify exact amino acid residues modified in a specified protein.
💠PTM Composition and Type Analysis: Determine the nature of the modification (e.g. mono- vs multi-phosphorylation, acetylation vs methylation).
💠Site-Specific Quantification: Measure modification occupancy and compare across conditions or production batches.
💠Comparative PTM Profiling: Assess differences in modification patterns due to treatment, mutation, or expression system.
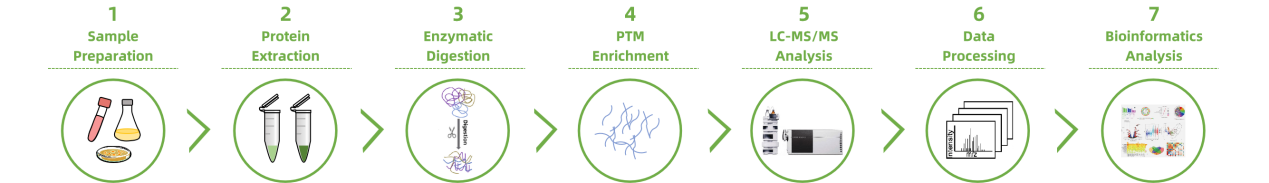
Workflow of Targeted Post-Translational Modification (PTM) Profiling Service
Why Choose MtoZ Biolabs
☑️High Sensitivity and Specificity: Advanced instrumentation and enrichment yield detection of low-abundance modified peptides.
☑️Site-Level Resolution: Precise assignment of modification sites avoids ambiguity and supports structural or functional interpretation.
☑️Custom Workflow Design: Tailored analytical plans to the protein, modification type, and project goals.
☑️Expert Scientific Support: Experienced analysts ensure data quality, interpretation, and actionable conclusions.
☑️Reproducible and Regulatory-Ready: Standardized protocol, quality control, and clear documentation support publication or compliance needs.
Applications of Targeted Post-Translational Modification (PTM) Profiling Service
-
Characterization of therapeutic antibodies and recombinant proteins for key PTMs that affect function and quality.
-
Monitoring PTM profiles in biosimilar development to ensure equivalence with reference products.
-
Evaluation of PTM changes in proteins produced under stress, mutation, or process variation.
-
Support of mechanistic studies linking specific PTMs to protein activity, localization, or disease pathways.
-
Quality control of protein lot-to-lot consistency by confirming modification status and site occupancy.
FAQ
Q1: What types of samples are suitable?
We accept a wide range of protein and biological samples, including purified proteins, recombinant proteins, antibodies, and other therapeutic proteins. Cell lysates, tissues, and biological fluids can also be submitted, with MtoZ Biolabs performing target protein extraction and preparation.
Q2: How should I prepare my samples?
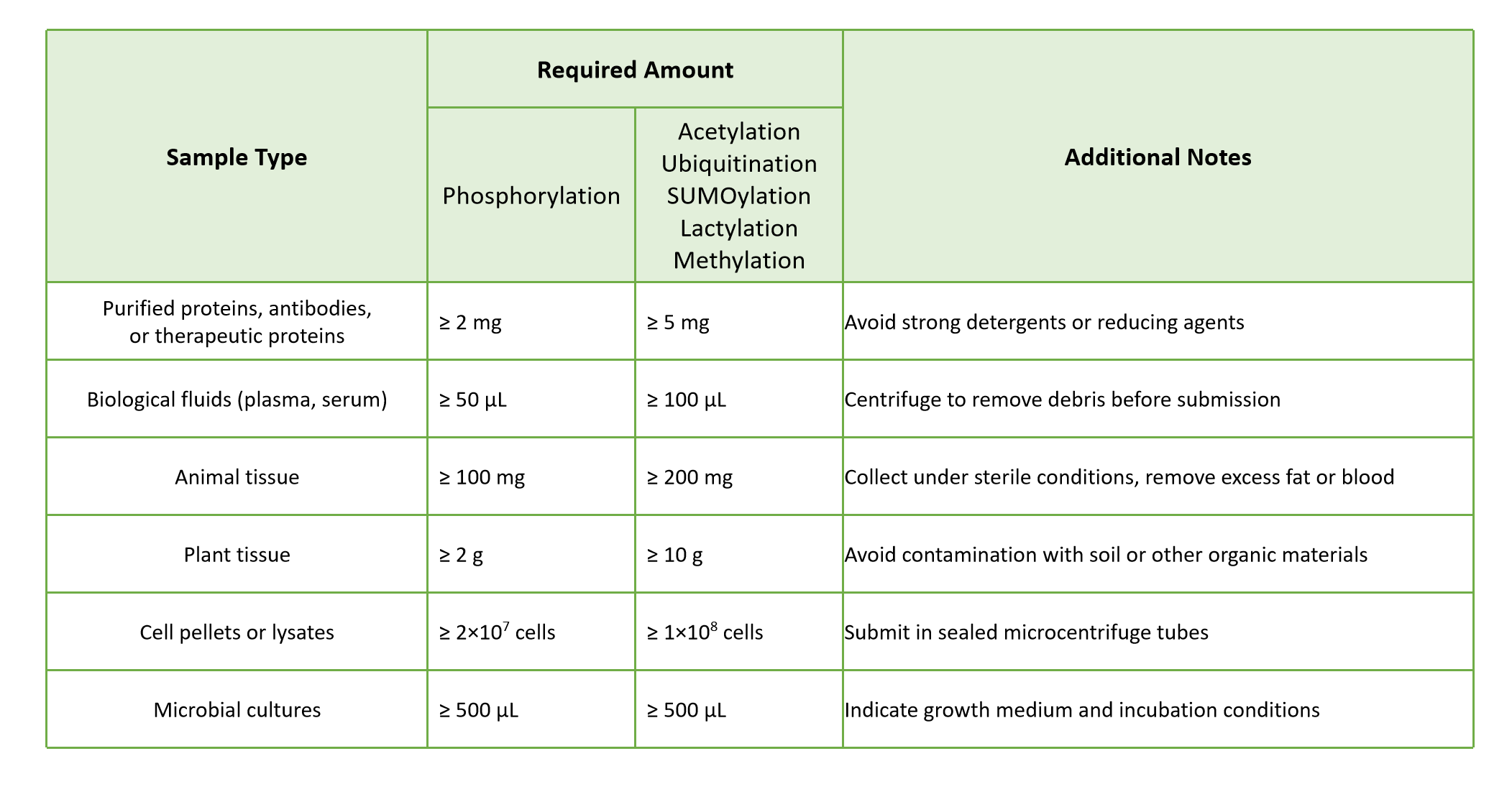
- All samples should be stored at –80°C.
- Use dry ice or ice packs during shipment to preserve protein integrity.
- Avoid freeze–thaw cycles that may alter disulfide linkages.
For more information, please refer to Sample Submission Guidelines for Proteomics.
Q3: What is the service general workflow?
Q4: What data formats are provided?
Results are delivered in standard and easy-to-access formats, including:
-
Raw MS data files (.raw, .wiff, .d)
-
Processed data and quantification tables (.xlsx, .csv)
-
Spectral and chromatogram images (.pdf, .png)
-
Final analytical report (.pdf)
All deliverables are compatible with common data analysis software. Additional file formats or data structures can be provided upon request to meet specific research or publication requirements.
Start Your Project with MtoZ Biolabs
Contact us to discuss your experimental design or request a quote. Our technical specialists are available to provide a free business assessment.







